Abstract
Introduction: Despite frontline response rates of 65-70% with AZA-VEN a majority of pts relapse, with only 35-40% alive at 3+ years and pts with TP53 mutation (TP53mut) have continued poor outcomes with median overall survival (mOS) of 5-6 months (m) (Kim K et al, Cancer 2021, Pollyea D et al ASH 2021). R/R AML have mOS of around 6 m with HMA-VEN. Magro, an anti-CD47 antibody that blocks the "don't eat me signal" on leukemia cells, demonstrated efficacy with AZA in ND TP53wt (ORR: 63%, mOS 18.9 m) and TP53mut AML (ORR 49%, CR 33%, mOS 10.8m) (Daver N, EHA 2022). Magro with AZA-VEN increased phagocytosis in AML cell lines in vitro, and prolonged survival in vivo in AML PDXs regardless of TP53 mut status (Jia Y et al, ASH 2021). We designed a phase Ib/II clinical trial to evaluate this triplet combination therapy (NCT04435691).
Methods: Pts ³18 years with ECOG PS ≤2, WBC <15x109/L and adequate organ function were eligible. The initial phase Ib enrolled R/R AML pts only. Once the RP2D was established the Phase II allowed both frontline and R/R pts. The frontline cohort enrolled pts ≥75yrs, or pts with documented comorbidities conferring ineligibility for intensive therapy; or pts with adverse risk karyotype (defined by ELN 2017 criteria) and/or TP53mut regardless of age/fitness.
Pts received AZA 75 mg/m2 on D1-7, VEN 400 mg (or equivalent per VEN label) on D1-28. The Magro RP2D dose was established as 1 mg/kg on C1D1 and C1D4, 15 mg/kg on C1D8, and 30 mg/kg on C1D11, C1D15, C1D22, weekly in cycle 2, and every 2 weeks in cycle 3+.
Primary study objectives were safety and RP2D of the triplet. Secondary objectives included CR/CRi rate, DOR and OS. Responses were per ELN2017 criteria.
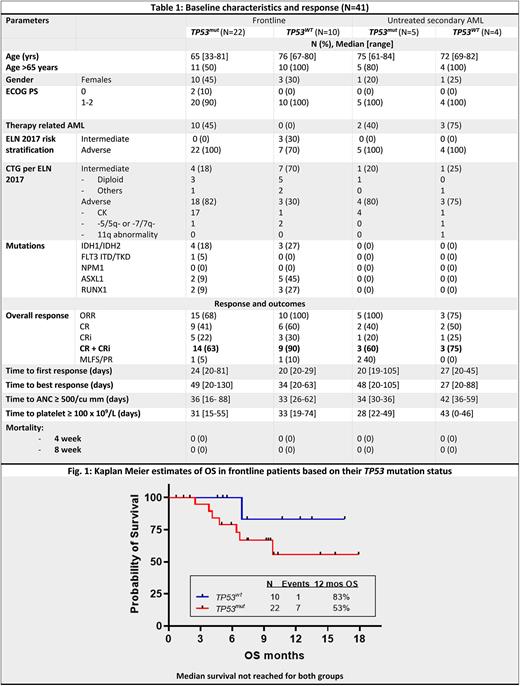
Results: From Aug 2020 to data cut off on June 1 2022, 74 pts were enrolled of whom 41 pts had ND AML (32 de novo or therapy related, 9 untreated secondary). An additional 4 pts had prior HMA treated, secondary ND AML. 29 pts had R/R disease (12 prior VEN naïve and 17 prior VEN exposed). Table 1 shows the baseline characteristics and responses in the ND AML pts based on their TP53 mutation status. Majority of the ND pts had high risk disease: 38/41 (93%) pts had adverse risk per ELN 2017, including adverse cytogenetics in 29/41 (71%) pts and therapy related AML in 15/41 (37%) pts. All pts who received at least one dose of any study drug were included for response and adverse events (AEs).
In the ND cohort, 33/41 pts (80%) had an overall response, including in 27 TP53mut (ORR 74%, CR/CRi 63%, CR 41%) and 14 TP53wt pts (ORR 93%, CR/CRi 86%, CR 57%).With a median (med) follow up (F/U) of 9.2 m for the frontline non-secondary ND group (n=32), the mOS was not reached (NR) for both TP53mut and TP53wt pts (1 year OS: 53% and 83%, resp, Fig. 1) with med DOR 7.4 m and NR, resp. In the ND untreated secondary AML pts, with a med F/U of 17.5 mo, the med DOR and OS were 6.5 and 7.2 m respectively. 8/27 (30%) TP53mut and 4/14 (29%) TP53wt proceeded to allogeneic stem cell transplantation (ASCT) in remission.
In the R/R cohort, only 2/17 (12%) pts with prior VEN exposure had a response (both CRi) with DOR 3.6 and 2.5m and mOS of 3.1 m. This cohort has been closed for futility. 8/12 (75%) VEN-naïve pts responded (6 CR, 1 CRi and MLFS each) with a med DOR and med OS of 5.1 and 7.4 m respectively with med F/U 14 mo. 4/12 (33%) VEN-naïve R/R pts could proceed directly to a ASCT, with a mOS of 10 m in transplanted pts.
The 4- and 8-weeks mortality across the whole cohort was 0% and 7% respectively (all in the R/R pts with no 8-wk mortality in ND pts). Eighteen pts (24%) had a ≥ grade 3 anemia while on study. With pre-Magro dosing Hb cut-off at 8.5 g/dL there were no anemia related life-threatening events or deaths. The med drop in Hb post first infusion of Magro in the N/D cohort (n=41) was 1.2 g/dl (range, 0 - 3.9 g/dl). Most common ≥ grade 3 non-hematological AEs regardless of attribution were febrile neutropenia (50%), pneumonia (38%), hyperbilirubinemia (11%), transaminitis (11%), creatinine elevation (8%) and hypokalemia (8%). No immune related AEs were documented.
Conclusion: The triplet combination of AZA VEN Magro appears safe with encouraging CR rates in a cohort of ND pts with 93% adverse risk ELN and 71% with adverse cytogenetic features. Responses in R/R AML were modest with prior VEN exposed pts faring poorly. Further trial enrollment and correlative analysis is underway and a phase III placebo controlled, randomized, international study to evaluate this triplet in ND AML has been initiated (ENHANCE-3, NCT05079230).
Disclosures
Daver:Agios, Celgene, SOBI and STAR Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kartos and Jazz Pharmaceuticals: Other: Data monitoring committee member; Karyopham Therapeutics and Newave Pharmaceutical: Research Funding; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Novartis, Jazz, Amgen, Servier, Karyopharm, Trovagene, Trillium, Syndax, Gilead, Pfizer, Bristol Myers Squibb, Kite, Actinium, Arog, Immunogen, Arcellx, and Shattuck: Consultancy, Other: Advisory Role; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Gilead, Immunogen, Pfizer, Bristol Myers Squibb, Trovagene, Servier, Novimmune, Incyte, Hanmi, Fate, Amgen, Kite, Novartis, Astex, KAHR, Shattuck, Sobi, Glycomimetics, Trillium: Research Funding. Loghavi:Astellas: Research Funding; PeerView: Honoraria; Amgen: Research Funding; QualWorld: Consultancy; GLG: Consultancy; Abbvie: Consultancy, Current equity holder in publicly-traded company. Kadia:Novartis: Consultancy; cellenkos: Research Funding; Servier: Consultancy; Genfleet: Research Funding; Regeneron: Research Funding; Ascentage: Research Funding; Astellas: Research Funding; cyclacel: Research Funding; Amgen: Research Funding; Genentech: Consultancy, Research Funding; JAZZ: Consultancy, Research Funding; Pfizer: Research Funding; Astex: Honoraria; Delta-Fly: Research Funding; PinotBio: Consultancy; Iterion: Research Funding; Glycomimetics: Research Funding; BMS: Consultancy, Research Funding; AstraZeneca: Research Funding; Agios: Consultancy; Abbvie: Consultancy, Research Funding. DiNardo:Jazz: Honoraria; Astellas: Honoraria; Gilead: Honoraria; Kura: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Research Funding; Forma: Research Funding; Cleave: Research Funding; Astex: Research Funding; Takeda: Honoraria; Novartis: Honoraria; LOXO: Research Funding; ImmuneOnc: Honoraria, Research Funding; Foghorn: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Bluebird Bio: Honoraria; Servier: Consultancy, Honoraria, Research Funding; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; GenMab: Membership on an entity's Board of Directors or advisory committees. Pemmaraju:stemline: Consultancy; abbvie: Consultancy; immunogen: Consultancy; mustangbio: Research Funding; incyte: Consultancy; novartis: Research Funding; pacylex: Consultancy, Research Funding; samus: Research Funding; daiichi sankyo: Research Funding; cellectis: Research Funding; cellularity: Research Funding. Jabbour:Pfizer: Other: Advisory Role, Research Funding; Amgen: Other: Advisory Role, Research Funding; Bristol Myers Squibb: Other: Advisory Role, Research Funding; Genentech: Other: Advisory Role, Research Funding; Spectrum: Research Funding; Adaptive Biotechnologies: Other: Advisory Role, Research Funding; AbbVie: Other: Advisory Role, Research Funding; Takeda: Other: Advisory Role, Research Funding. Sasaki:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees; Otsuka Pharmaceuticals: Honoraria; Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees. Borthakur:Astex Pharmaceuticals, Ryvu, PTC Therapeutics: Research Funding; Pacylex, Novartis, Cytomx, Bio Ascend: Membership on an entity's Board of Directors or advisory committees; Catamaran Bio, Abbvie, PPD Development, Protagonist Therapeutics, Janssen: Consultancy. Yilmaz:Pfizer: Research Funding; Daiichi-Sankyo: Research Funding. Issa:Novartis, Kura Oncology, Nuprobe: Consultancy; Celgene, Kura Oncology, Syndax, Merck, Cullinan and Novartis: Research Funding. Andreeff:AstraZeneca: Research Funding; German Research Council: Membership on an entity's Board of Directors or advisory committees; Oncolyze: Current holder of stock options in a privately-held company; CLL Foundation: Membership on an entity's Board of Directors or advisory committees; Chimerix: Current holder of stock options in a privately-held company; Glycomimetics: Consultancy; Aptose: Consultancy, Membership on an entity's Board of Directors or advisory committees; Leukemia & Lymphoma Society: Membership on an entity's Board of Directors or advisory committees; Medicxi: Consultancy; Cancer UK: Membership on an entity's Board of Directors or advisory committees; Breast Cancer Research Foundation: Research Funding; NCI: Membership on an entity's Board of Directors or advisory committees; Oxford Biomedical UK: Research Funding; Daiichi-Sankyo Inc.: Consultancy, Research Funding; Pinot Bio: Research Funding; Brooklyn ITX: Research Funding; Senti Bio: Consultancy, Research Funding; Kintor Pharmaceutical: Research Funding; Reata: Current holder of stock options in a privately-held company; Syndax: Consultancy, Research Funding. Ravandi:Amgen: Honoraria, Research Funding; AstraZeneca: Consultancy; Xencor: Research Funding; Prelude: Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Novartis: Consultancy; Astex/Taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS/Celgene: Consultancy, Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Syos: Consultancy, Honoraria, Research Funding; Astellas: Consultancy, Honoraria, Research Funding; Biomea Fusion, Inc.: Research Funding. Garcia-Manero:Genentech: Honoraria, Research Funding; Gilead Sciences: Research Funding; Curis: Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Astex: Consultancy, Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Aprea: Honoraria; Acceleron Pharma: Consultancy. Kantarjian:Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; Ipsen Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Astellas Health: Honoraria, Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Consultancy, Research Funding; KAHR Medical Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Research Funding; NOVA Research: Honoraria; ImmunoGen: Research Funding; Jazz Pharmaceuticals: Research Funding; Pfizer: Honoraria, Research Funding; Takeda: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal